What is Water Hardness? What are its causes, effects, and control measures in the industrial, commercial, agricultural, and domestic water infrastructure?
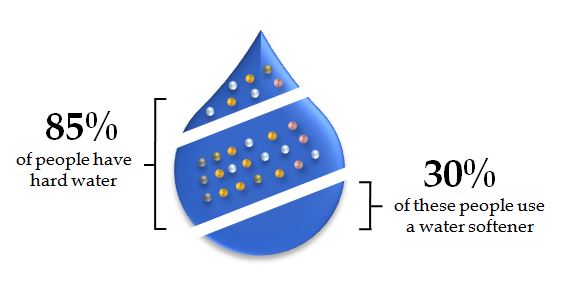
Water hardness is a common water quality issue that arises from the presence of dissolved minerals, primarily calcium and magnesium, in water. It is typically measured in terms of calcium carbonate (CaCO3) equivalents, expressed as parts per million (ppm) or milligrams per liter (mg/L).
Water hardness is categorized into two types: temporary hardness and permanent hardness.
- Temporary Hardness: Temporary hardness is caused by the presence of dissolved bicarbonate minerals, such as calcium bicarbonate (Ca(HCO3)2) and magnesium bicarbonate (Mg(HCO3)2), in water. When water is heated or exposed to air, these bicarbonate minerals decompose, releasing carbon dioxide gas and forming insoluble calcium or magnesium carbonate. This process is known as “calcium carbonate precipitation” or “scaling,” which leads to the formation of deposits in pipes, boilers, and other equipment.
Examples of temporary hardness include:
- When hard water is boiled, you may observe the formation of a white or off-white scale on the surface of kettles, teapots, and water heaters. This scale is composed of calcium carbonate or magnesium carbonate deposits.
- Scaling in coffee machines, espresso makers, and other appliances that use hot water can occur due to the precipitation of calcium and magnesium carbonates.
The chemical reactions involved in the formation of scale deposits due to temporary hardness can be represented as follows:
Ca(HCO3)2 → CaCO3 + CO2 + H2O
Mg(HCO3)2 → MgCO3 + CO2 + H2O
- Permanent Hardness: Permanent hardness is caused by the presence of dissolved sulfates, chlorides, and nitrates of calcium and magnesium in water. Unlike temporary hardness, permanent hardness cannot be removed by boiling the water. Instead, it requires specialized treatment methods to be effectively reduced.
Examples of permanent hardness include:
- When water contains high levels of calcium sulfate (CaSO4) or magnesium sulfate (MgSO4), it can lead to the formation of scale deposits on surfaces and within pipes. This can result in reduced water flow, clogging, and decreased efficiency of industrial equipment, such as heat exchangers and boilers.
- Agricultural operations relying on irrigation systems may experience issues with scaling and reduced water flow due to permanent hardness. The accumulation of mineral deposits can affect the performance of sprinklers and result in uneven water distribution across fields.
- In households, permanent hardness can cause scaling and clogging of plumbing fixtures, reducing water flow in taps and showers. It can also impact the effectiveness of water-using appliances like washing machines, dishwashers, and water heaters, leading to increased maintenance and energy costs.
The chemical reactions involved in the formation of permanent hardness are:
CaSO4 → Ca2+ + SO4^2-
MgSO4 → Mg2+ + SO4^2-
Causes of Water Hardness:
- Geological Sources: Water hardness is often influenced by the geological composition of the area. Water passing through mineral-rich geological formations, such as limestone or gypsum, tends to acquire higher levels of hardness minerals.
- Industrial and Agricultural Activities: Certain industrial processes and agricultural activities can contribute to water hardness. For example, mining operations, manufacturing plants, and agricultural runoff can introduce minerals into water sources, increasing water hardness.
Effects of Water Hardness:
- Industrial Sector: In industrial settings, water hardness can lead to several problems. Scaling, caused by the deposition of minerals on pipes, boilers, heat exchangers, and other equipment, reduces efficiency and necessitates frequent cleaning and maintenance. It can result in decreased heat transfer, increased energy consumption, and increased operational costs.
- Commercial Sector: In commercial establishments like hotels, restaurants, and laundries, water hardness can negatively impact various operations. It can cause stains on fixtures and surfaces, reduce the effectiveness of cleaning agents, and affect the quality and appearance of laundered items.
- Agricultural Sector: Water hardness can impact agricultural practices by affecting soil fertility and crop growth. Irrigation with hard water can lead to the accumulation of minerals in the soil, affecting its structure and nutrient availability. Additionally, hard water can reduce the effectiveness of fertilizers and pesticides, impacting agricultural productivity.
- Domestic Sector: In households, water hardness can create issues such as scale buildup in plumbing fixtures, reduced effectiveness of soaps and detergents, and the formation of soap scum on bathroom surfaces. It can also affect the performance and lifespan of appliances like water heaters, dishwashers, and washing machines.
Control Measures for Water Hardness:
- Water Softening: The most common method to control water hardness is through water softening techniques. Water softeners use ion exchange resins or other technologies to remove calcium and magnesium ions from water and replace them with sodium or potassium ions, effectively reducing water hardness. This method is commonly used in both industrial and domestic settings. This process can be implemented using various devices, such as water softener tanks or cartridge-based systems.
- Reverse Osmosis: Reverse osmosis (RO) systems can also effectively reduce water hardness. RO systems use a semipermeable membrane to remove dissolved minerals and impurities including calcium and magnesium from water, producing softened water as a result.
- Chelation: Chelating agents can be used to bind with the hard minerals in the water, preventing them from causing scaling and other issues. These agents form soluble complexes with the minerals, keeping them in suspension and minimizing their adverse effects.
- Chemical Softening or Lime-Soda Ash Softening: Chemical softening involves adding chemicals like lime (calcium hydroxide) or soda ash (sodium carbonate) to the water, which react with calcium and magnesium ions to form insoluble precipitates that can be removed through filtration.
- Electromagnetic Water Conditioners: These devices use electromagnetic fields to alter the physical properties of dissolved minerals, reducing their tendency to form scale deposits. However, their effectiveness in controlling water hardness is still a subject of debate and may vary based on water composition and application.
- Instant Solvent Softener (ISS): The Instant Solvent Softener (Swachh Paani) is a water conversion device that prevents hard water scale build-up and hard water stains when installed and gradually removes hard water scale from previously untreated older systems. ISS is a patented product and an instant water softener and water converter without chemicals and maintenance requirements. It is successfully operating in industrial and domestic applications in Anti-Scaling and De-Scaling in Cooling Towers, Condensers, Chilling Plant, Boilers, Humidifiers, Pipelines, Machinery, Water Pipelines, Faucets, Geysers, Dishwashers, Washing Machines, etc.
It’s important to note that the choice of control measures should be based on the specific requirements and characteristics of the water source, as well as the intended application. Consulting with water treatment experts or professionals can help determine the most suitable approach for controlling water hardness in each sector.

